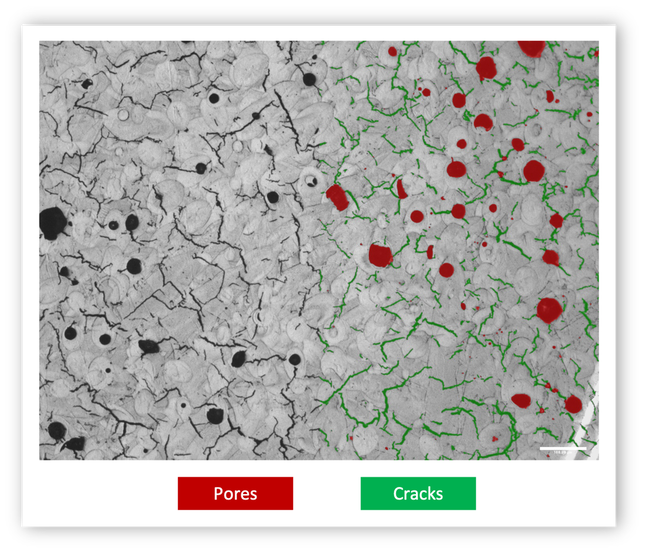
Porosity Analysis: Understanding Pore Size Distribution
No solid is entirely dense. All materials, from advanced ceramics to heavy metal alloys, contain channels or cavities which influence physicochemical reactions with gases or liquids. The measure of this empty space is generally defined as porosity. It can usually be expressed as a volume fraction or percentage, but determining the total void space in a solid can be deceptively challenging.
Laying the Groundwork for Pore Size Distribution Analysis
Not all pores are accessible from the surface, existing as closed internal cells. Carrying out closed porosity analysis via conventional methods like mercury intrusion is impossible as they rely on permeation to measure internal empty space. Additionally, some continuous cavities may only be accessible by size-restricted channels which can inhibit fluidic/gas intrusion. Mercury is unable to penetrate micropores with a nominal diameter of 3 nanometres (nm) or less, for instance.
Gas sorption is the preferred technique for measuring pore width at the Angstrom (Å) scale, with carbon dioxide (CO2) representing the ideal probe gas for micropores with diameters of 10Å and lower. These different characterizations (i.e. micro- versus macropores) are important as a simplistic total porosity measurement, as in the void fraction, offers very little information about the representative mean pore size distribution.
Pore size distribution, sometimes known as differential pore volume distribution (cm3/g), gives a spectrum of porosity based on the relative abundance of voids on the micro-, meso-, and macropore range. It is a more valuable metric than total porosity as it can provide detailed insights into more complex properties such as localized density. This is can be extremely useful in defect analysis, and quality assurance where powder uniformity is a critical parameter.
For example: Additive manufacturing methods like powder bed fusion rely on high uniformity feedstocks to form a final net shape via laser-induced sintering. A laser selectively irradiates powder material, causing particles to melt and fuse together. This is a form of atomic diffusion where the powder surface is gradually eliminated as the material densifies, eventually causing complete pore closure. Powder inhomogeneities would affect the amount of energy needed to incite boundary diffusion, and thus could lead to product defects or failure.
Pore Size Distribution Analysis with MIPAR